We are thrilled to announce that Dr. Steven Herbst, Central Indiana Orthopedics foot and ankle surgeon, recently performed the first BioPoly® Great Toe surgery in the United States. The surgery was performed on September 30 at Central Indiana Orthopedics Muncie Surgery Center.
Dr. Herbst partnered with BioPoly LLC to create an orthopedic surgical implant for great toe (big toe) surgery. “Product development has been a passion of mine for a long time,” Dr. Herbst said. “BioPoly brings engineering expertise and an innovative material to a joint in the foot that needs a better surgical answer. I am confident this implant will bring long-term relief to my patients suffering from arthritis of the big toe, and I look forward to providing that relief!”
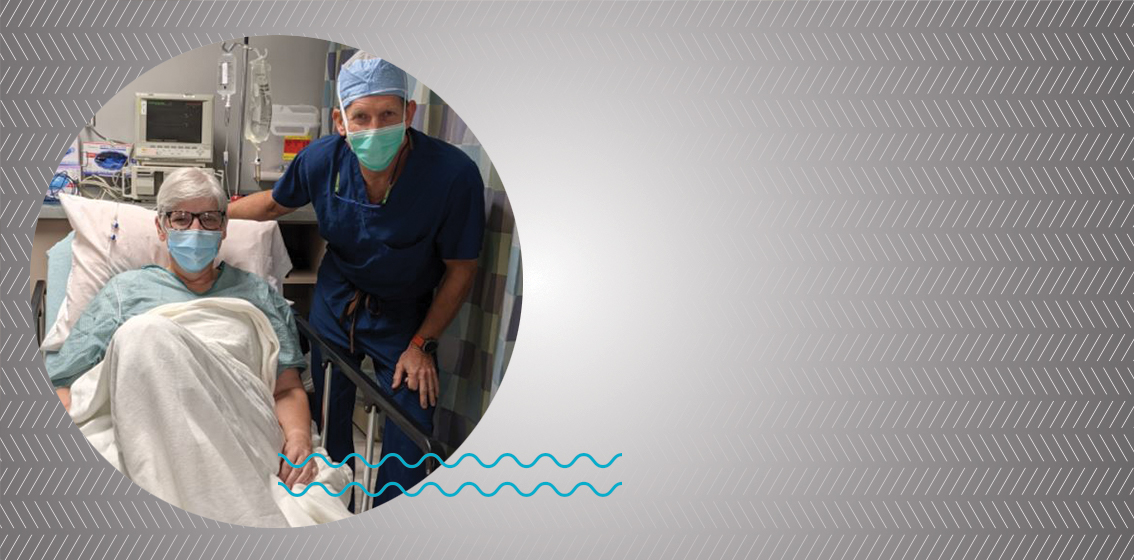
When Dr. Herbst met patient Laura High, she had arthritis in her big toe and was hoping for pain relief. After discussing both non-surgical and surgical treatment options, Laura decided surgery was the best treatment for her. Dr. Herbst shared with her that BioPoly would soon be releasing an innovative great toe implant for treatment of arthritis. Laura was confident that she wanted to proceed with surgery and wait for the BioPoly implant to be released to the market.
“The BioPoly Great Toe implant addresses a large unmet need in the market, and we are thrilled to have it available in the U.S. now. This small implant has a lot of technology packed into it and solves many of the issues that have caused other great toe implants to fail,” said Herb Schwartz, President of BioPoly.
The BioPoly® Great Toe implant is intended to restore the articular surface of the first metatarsal bone, the bone in the foot just behind the big toe. Patients with degenerative and post-traumatic arthritis in the first metatarsal joint could be a candidate for this surgical implant.
The implant is made of BioPoly’s biomaterial that is a combination of polyethylene and hyaluronic acid, thus creating a self-lubricating polymer with enhanced wear properties. Strong fixation to the bone is provided by a titanium stem which has a porous metal surface called OsteoSync™ (Sites Medical), a proven material that has been used successfully used in many other orthopedic implant applications.
BioPoly has been implanting their knee devices in Europe since 2012, but this great toe surgery is the first U.S. surgery for BioPoly and a big accomplishment. With the first FDA-cleared BioPoly device now available in the U.S., many more BioPoly implant applications are expected soon, according to the Company.
As for Laura, she couldn’t be happier with her surgical outcome. “Dr. Herbst & BioPoly are my perfect dream team,” Laura said. “My experience couldn’t have been any better. Thank you BioPoly and Central Indiana Orthopedics!”

BioPoly Engineer Stone Miguel, BioPoly Owner Herb Schwartz, Dr. Steven Herbst, Patient Laura High and BioPoly Molding Process Lead Steven High
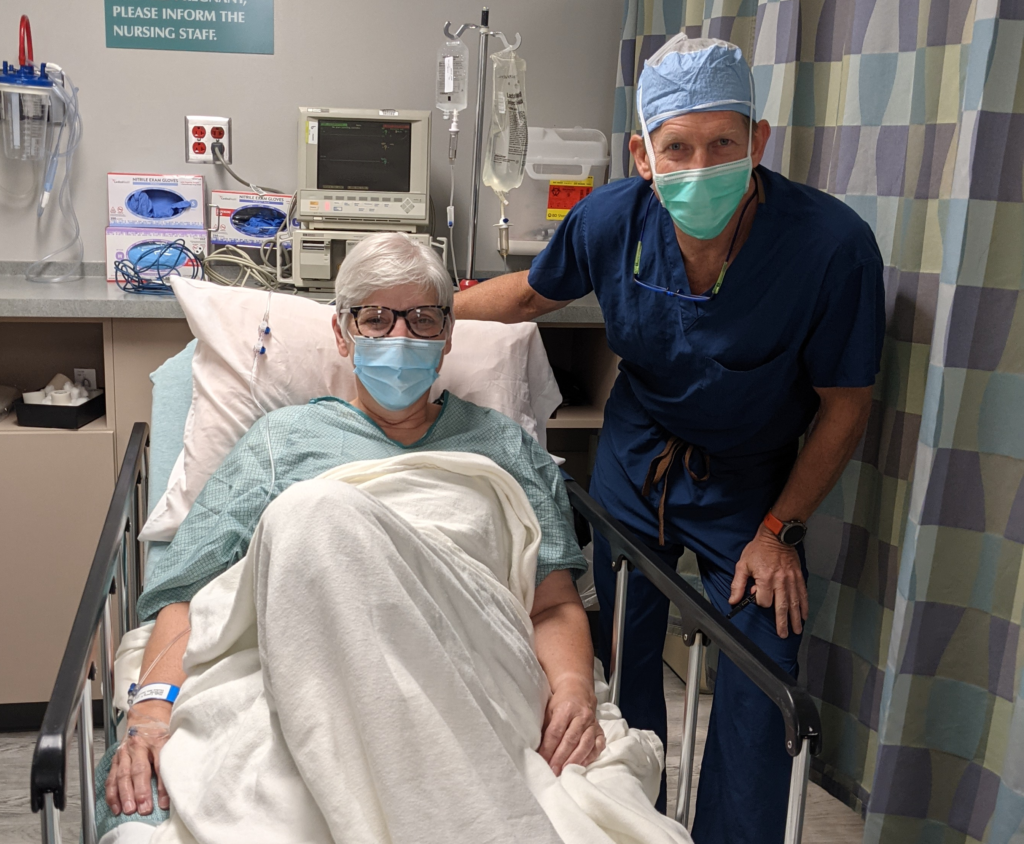
Laura and Dr. Herbst before surgery
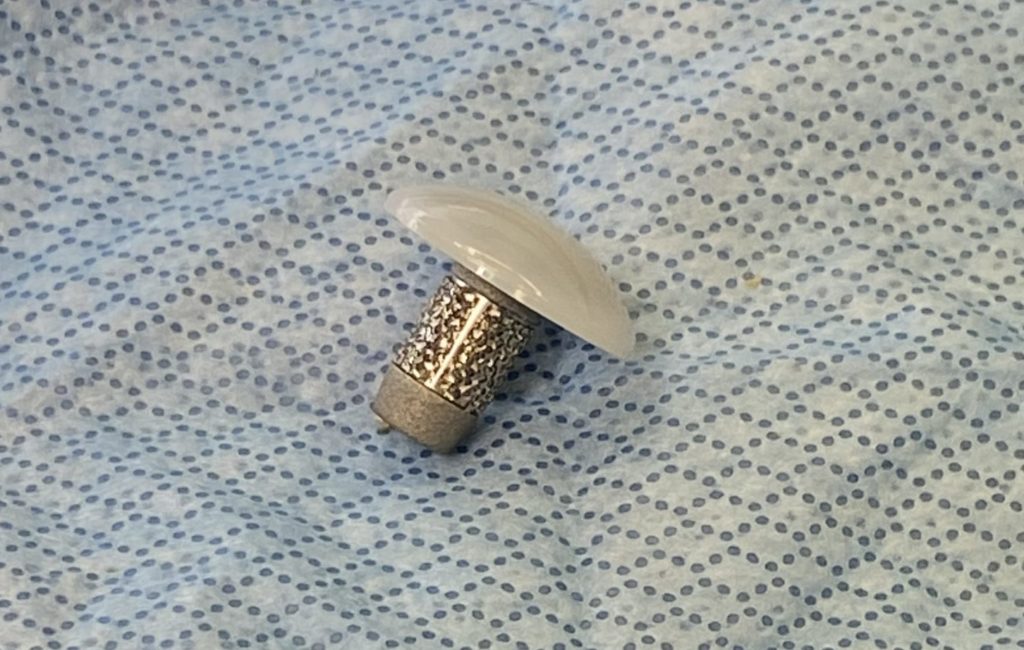
BioPoly® Great Toe Implant
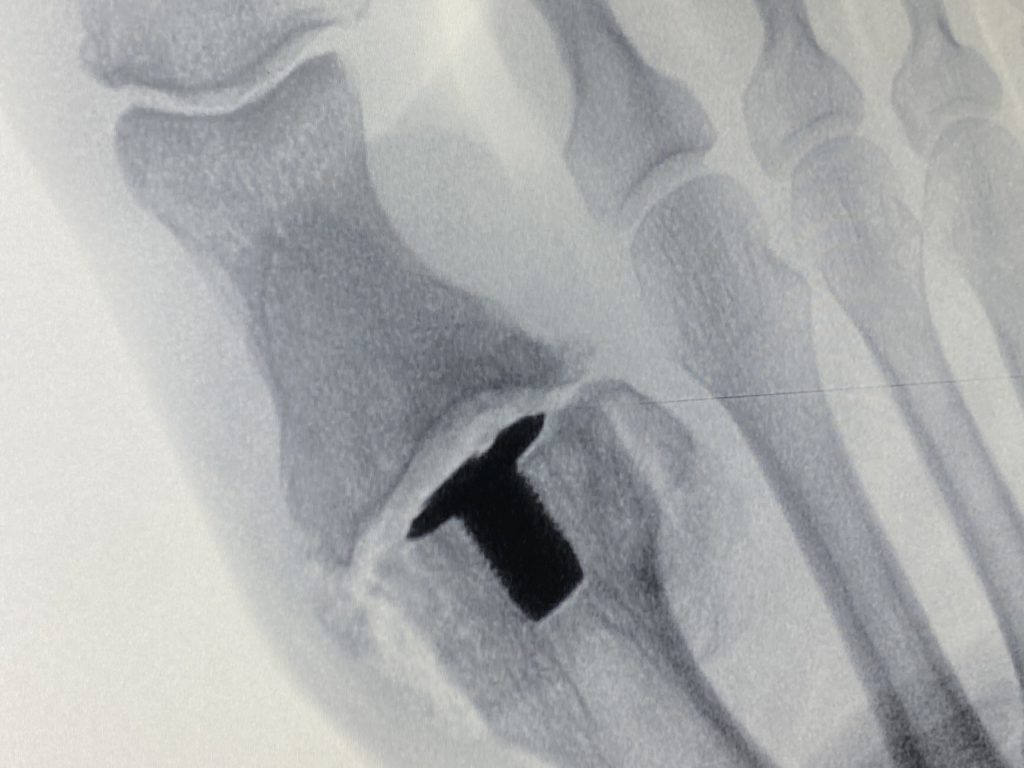
BioPoly® Great Toe Implant after surgery (x-ray)